Your safety at your fingertips. Our Gamot Authenticator Application (GamAuth) uses advanced technology to ensure that the over-the-counter medicine that you purchase conforms with the Food and Drugs Administration standards. With a simple app installation in your mobile phones, we can guarantee a high accuracy authentication that you can carry anytime, anywhere.
Our Gamot Authenticator Application – your friendly medicine checker!
– BitWagon
The Problem
The ease of access to the internet has made medicines more purchasable and easier to obtain than ever, but it has also made it more difficult for consumers to know if what they are buying is counterfeit, contaminated, or otherwise unsafe.
According to Philippine Republic Act No. 8203 or the Special Law on Counterfeit Drugs (Kintanar & Noriega-Reodica, 1996), Counterfeit drug/medicine refers to medicinal products with the correct ingredients but not in the amounts as provided hereunder, wrong ingredients, without active ingredients, with insufficient quantity of active ingredient, which results in the reduction of the drug’s safety, efficacy, quality, strength or purity. It is a drug that is deliberately and fraudulently mislabeled concerning identity and/or source or with fake packaging and can be applied to both branded and generic products. Counterfeit Drugs shall refer to the drug itself or its container, labeling, or any part of them, bearing unauthorized trademarks or trade names that resemble registered marks of another entity. It also includes drugs refilled in containers with legitimate labels by unauthorized individuals, unregistered imported drug products (except for personal use with accompanying medical records), and drugs with no amount of the claimed active ingredient, a different active ingredient, or less than eighty percent of the stated active ingredient. These counterfeit drugs are distinct from adulterated drugs, which may have reduced efficacy due to expiration.
In the United Nations Office on Drugs and Crime or UNODC report (Bandiola, 2019), it is said that 460 pharmaceutical crime incidents took place within Southeast Asia from 2013 to 2017 while a total of 213 incidents occurred outside the region. Out of these 460 incidents, 193 of which occurred in the Philippines, 110 in Thailand, 93 in Indonesia, and 49 in Vietnam.
During the Covid-19 pandemic, we encountered a shortage of flu-related over-the-counter medicines. Consequently, this shortage led to an abundance of non-FDA and non-DTI-approved pharmaceutical products over the internet and on the streets.
In January and March 2022, there were two known major police operations wherein PHP 30 million and PHP 3.5 million worth of counterfeit medicines were seized in Parañaque City (Patinio, 2022) and Ozamiz City (Jerusalem, 2022). Having these events happened in the same year with a voluminous amount of counterfeit medicines shows that this is an ongoing and pressing problem and issue in society.
As of January 2023, there is no known accessible tool that will help the consumer in determining the authenticity of the pharmaceutical product even after they purchased it. Hence, putting major health threats in every “Juan”. Having a tool to help the consumers will make the fight against counterfeit products more effective, similar to the goals and objectives of the FDA advisories related to this. These counterfeit medicines pose a serious threat to public health and safety. Their effectiveness is unverified and questionable. They may worsen illness and disease. They may cause serious adverse health consequences, another disease, drug resistance, or worse, death.
With the help of Machine Learning and Data Science, our study aimed to empower the consumers in determining whether the over-the-counter medicines they will purchase are DTI-registered and FDA-approved or not by simply scanning their packaging using their smartphone.
Problem Validation and Result
In February 2022, due to the ongoing risk of the COVID-19 pandemic, our group chose to conduct a survey via Google form (APPENDIX A). The purpose of the survey was to determine the following:
- Does the problem of unsafe medicine really exist?
- Is there already an existing solution to the problem?
- What are the current methods used by consumers to ensure the authenticity of their purchase?
- What are their preferences when it comes to finding a long-term solution to the problem?
The survey form is structured into five sections to gather information effectively. The first section, “Purpose of the Survey and Data Privacy Consent,” serves two main functions. It explains the purpose and objectives of the survey and ensures that respondents give their consent to use their limited information, including full name, age, and location, solely for study purposes.
The second section, “Respondent’s Information,” contains questions aimed at collecting demographic details from the participants. This section helps in understanding the characteristics of the respondents.
Moving on to the third section, “Details of their Last Pharmaceutical Purchase,” respondents are asked to provide information about their latest medicine purchase, along with the location of the purchase. This section aims to gain insights into the buying behavior and preferences of the participants.
In the fourth section, “Authenticity of the Medicine (Images),” respondents are presented with ten images of medicines. Their task is to identify which medicines they believe to be authentic. This section contributes to understanding consumer perceptions regarding pharmaceutical product authenticity.
Lastly, the fifth section, “Respondent’s Inputs and Ideas,” provides space for participants to share their valuable inputs and feedback on the identified problem. This section encourages respondents to offer suggestions, opinions, or any other relevant information that could contribute to the study’s objectives.
By dividing the survey into these distinct sections, the research team aims to collect comprehensive and structured data, enabling them to draw meaningful conclusions and insights from the respondents’ valuable input.
Survey Result
A total of 208 participants consisting of 105 males and 103 females responded to the survey. A majority of respondents fell within the age range of 21 to 40 years old and resided in the National Capital Region. Please refer to Table 1-1 for a comprehensive summary of the respondents’ demographics.
| Total Respondents | 208 | |
| Gender | ||
| Male | 50.49% | 105 |
| Female | 49.51% | 103 |
| Age Group | ||
| Below 20 | 15% | 31 |
| 21-40 | 72% | 149 |
| 41-60 | 13% | 26 |
| 61 and above | 1% | 2 |
| Residence | ||
| National Capital Region | 64% | 133 |
| Region IV-A: CALABARZON | 16% | 33 |
| Region I: Ilocos Region | 7% | 15 |
| Region III: Central Luzon | 4% | 8 |
| Others | 8% | 17 |
Participants were asked to provide information regarding their most recent medicine purchase, including the specific medicine they bought and the location of the purchase. This data allowed for a comprehensive understanding of the participants’ recent buying behaviors and provided valuable insights into their choices and preferences when it comes to medicines.
Contextual understanding. By asking about the respondents’ last medicine purchase, the researchers gained insights into the respondents’ recent experiences with purchasing medicines. This context provided valuable information regarding their familiarity with the process, their preferences for specific types of medicines, and their usual purchasing locations.
Identification of Potential Counterfeit Medicines. By collecting data on where the respondents purchased their last medicine, the researchers identified specific sources or types of outlets where counterfeit or substandard medicines may be prevalent. This information helped identify areas of concern and guide efforts to combat the circulation of counterfeit medicines.
Awareness of Authentication Practices. By understanding where the respondents obtained their last medicine, the researchers assessed their awareness of and adherence to authentication practices. This data highlighted gaps in knowledge or awareness, indicating areas where educational campaigns or interventions might be needed to promote the importance of verifying medicine authenticity.
Comparative Analysis. The data on respondents’ last medicine purchases was used for comparative analysis, allowing the researchers to examine differences in authentication practices among different demographic groups or geographic regions. This analysis provided insights into potential variations in medicine quality and awareness levels across various segments of the population.
Based on the data presented in Figure 1-1, it is evident that a significant majority of respondents reported purchasing their medicines from Big Pharmaceutical Outlet. Additionally, Table 1-2 highlights that Paracetamol, specifically the brand Biogesic, emerged as the most commonly purchased type of medicine among the respondents.
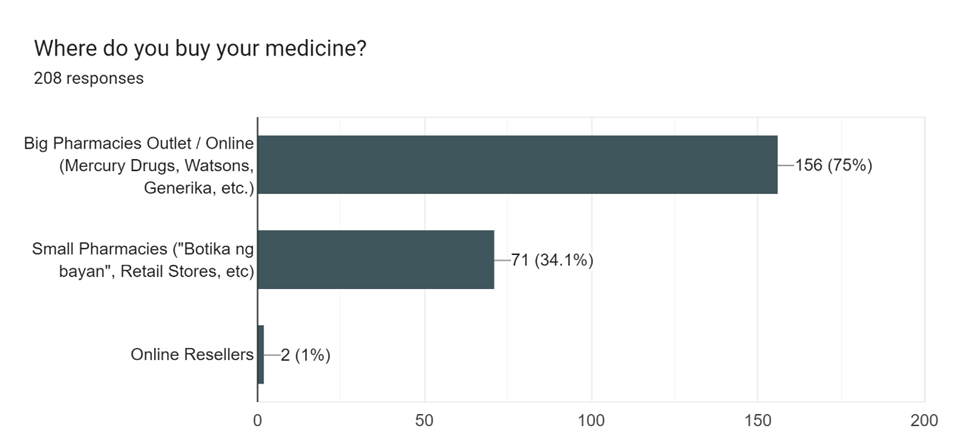
| Losartan | % of Total Count | Total Purchase |
| Biogesic | 18% | 38 |
| Paracetamol | 16% | 34 |
| Bioflu | 5% | 11 |
| Vitamin C/E | 5% | 10 |
| Ceterizine | 3% | 6 |
| Mefenamic Acid | 2% | 5 |
| Neozep | 2% | 5 |
| Lozartan | 2% | 4 |
| Saridon | 1% | 3 |
| Valparin | 1% | 2 |
| Others | 45% | 94 |
The respondents were also asked to identify whether the medicine shown was authentic or fake.
Assessing Awareness and Knowledge. By conducting such tests, the researchers gauged the respondents’ awareness and knowledge regarding medicine authentication. It helped determine if the respondents were familiar with the visual cues or indicators that distinguish genuine medicines from counterfeit ones as provided in the FDA advisories available online. This information was crucial in understanding the level of awareness and education needed to combat the problem of counterfeit medicines.
Evaluating the Effectiveness of Current Authentication Measures. Testing the respondents’ ability to identify authentic medicines provided insights into the effectiveness of existing authentication measures. It helped determine if the current mechanisms were successful in enabling consumers to differentiate between genuine and counterfeit products. If the respondents struggled to identify authentic medicines, it suggested a need for improved or more accessible authentication methods.
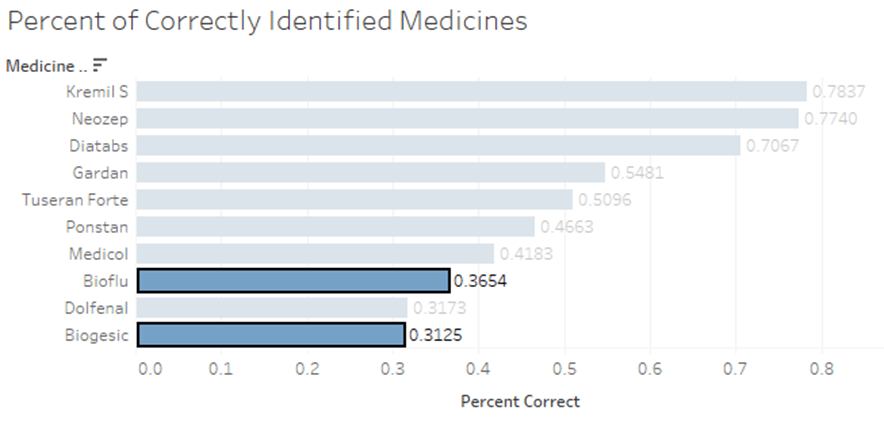
Based on the data presented in Figure 1-2, it is evident that Biogesic and Bioflu, which are among the most commonly purchased medicines, pose a challenge in terms of identifying their authenticity. The results indicate that only approximately 31% to 36% of the respondents were able to correctly determine whether these medicines were authentic or fake. This highlights the difficulty in distinguishing genuine products from counterfeits, emphasizing the need for improved authentication measures and consumer education in this particular context.
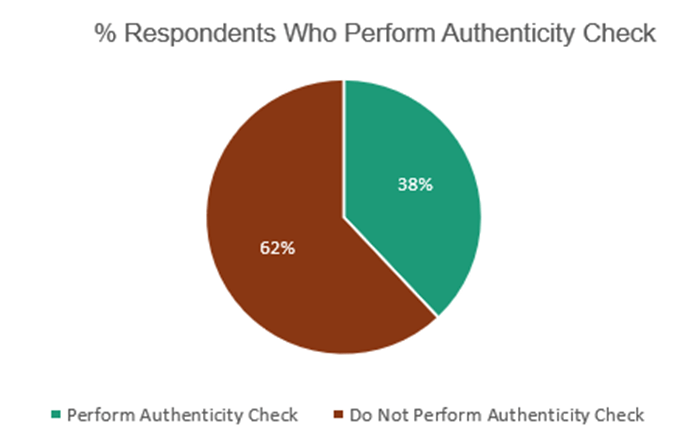
The findings presented in the preceding figures and tables offer valuable insights into the challenges surrounding medicine authentication, despite the availability of FDA advisories online. Moreover, the survey also explored whether respondents conducted authenticity checks when purchasing medicine. Regrettably, Figure 1-3 reveals that only 38% of the respondents actually performed authenticity checks, indicating a relatively low proportion of individuals taking proactive measures to verify the authenticity of the medicines they purchase. These results underscore the importance of raising awareness and promoting the practice of authenticity checks among consumers to ensure their safety and mitigate the risks associated with counterfeit medicines.
Shown in Figure 1-4 are the common words used by the participants, such as packaging, seal, patterns, compare, and trusted stores, which reflect their perceptions and suggestions for identifying the authenticity of medicines.
Packaging. Participants might be referring to specific features or characteristics of the medicine packaging that can help them determine its authenticity. This could include details like brand logos, fonts, colors, or specific labeling information that they associate with genuine products.
Seal. The mention of a seal suggested that participants believed an authentication feature like a tamper-evident seal could be used to verify the integrity of the medicine packaging. A seal acts as a physical barrier that indicates if the packaging has been tampered with, ensuring that the product is genuine and has not been compromised.
Patterns. Participants might be referring to distinctive patterns, designs, or markings on the packaging that serve as visual cues for identifying authentic medicines. These patterns could be unique to a particular brand or manufacturer and would be difficult for counterfeiters to replicate accurately.
Compare. Participants might have suggested comparing the suspected medicine with a known genuine product or reference sample. By comparing visual or physical attributes, such as color, texture, size, or shape, consumers could identify any discrepancies or inconsistencies that might indicate a counterfeit.
Trusted Stores. Participants might have expressed the importance of purchasing medicines from trusted and reputable sources. They might have recommended relying on well-known pharmacies, authorized retailers, or licensed distributors to ensure the authenticity and quality of the medicines they purchase.
Overall, these words reflect the participants’ understanding of potential indicators of medicine authenticity. Their suggestions highlight the significance of visual cues, physical features, and reliable sources to help consumers make informed decisions and avoid counterfeit or substandard medicines.

As shown in Figure 1-5, the common words used by participants such as “mobile application,” “scan,” “authentic,” and “identify,” indicate their suggestions for a solution to authenticate medicines.
Mobile Application. Participants were referring to the use of a smartphone application as a tool for medicine authentication. They suggested the development of an application that could be installed on mobile devices, allowing users to access authentication features and information conveniently.
Scan. Participants proposed the use of scanning technology, likely involving the camera on a mobile device, to scan or capture specific elements on medicine packaging. This could include barcodes, QR codes, or other visual codes that contain information about the authenticity of the product.
Authentic. The mention of “authentic” indicated that participants wanted a solution that could verify and confirm the legitimacy of the medicine. They were looking for a reliable method to distinguish genuine medicines from counterfeit or substandard ones.
Identify. Participants expressed the need for a solution that could help them identify whether medicine is authentic or not. They wanted a clear indication or result that confirms the authenticity of the product, providing assurance and confidence in their purchasing decisions.
In summary, the participants’ use of these words suggests their desire for a mobile application with scanning capabilities that can authenticate medicines, ensuring that they are genuine and safe for consumption. The solution they envision would allow users to scan medicine packaging, verify its authenticity, and obtain clear identification results through the application.

Market Analysis
To determine the target market, the researchers used the TAM-SAM-SOM methodology to streamline the potential users. Even though there are more than 7M Serviceable Obtainable Market, our target was to deploy the application in small groups. Our pilot market will be identified once we have a working minimum viable product (MVP).
TAM-SAM-SOM
Using the data from Statistica Research and Development (Philippine Statistics Authority (PSA), 2019), combined with the survey results, the target Serviceable Obtainable market is around 7M Filipino Smartphone users. TOM-SAM-SOM details are shown in Figure 2-1.
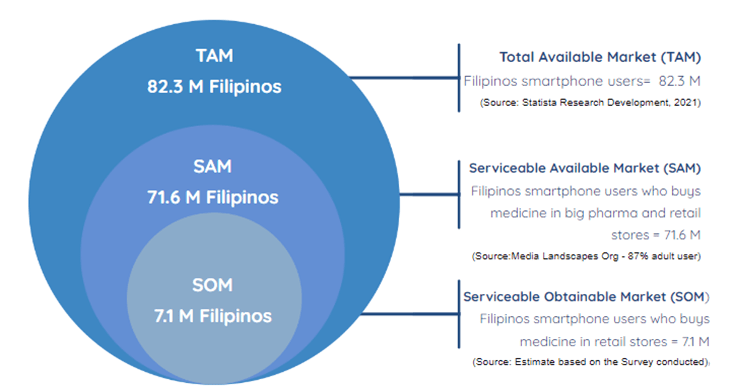
In market analysis, three fundamental market metrics are considered: Total Available Market (TAM), Serviceable Available Market (SAM), and Serviceable Obtainable Market (SOM).
TAM represents the overall market demand for a specific product or service, without any limitations or constraints. SAM, on the other hand, refers to the portion of the TAM that falls within the company’s reach and can be effectively targeted with its products or services. Lastly, SOM represents the specific segment of the SAM that the company can realistically capture or obtain, considering its available resources, competition, and capabilities.
By understanding and analyzing these market metrics, businesses can gain valuable insights into their potential market opportunities and devise strategies to optimize their market presence effectively.
Internal Analysis
Bring-Build-Buy Map
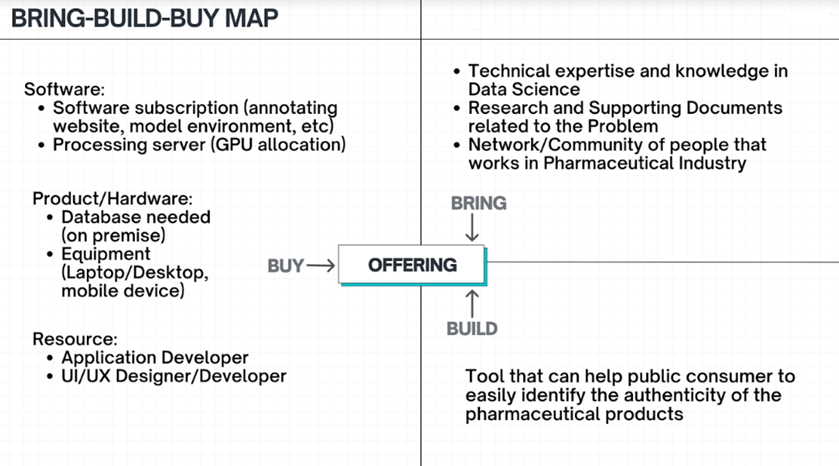
The Bring-Build-Buy Map shown in Figure 2-2 describes our assets and the things that we need to invest in. Technical expertise, research skills, and a network of people in the pharmaceutical industry are some of the things that our group can already utilize. In terms of investments, like any other application development process, we need to put resources into purchasing software and hardware products, and onboarding developers for mobile applications.
SWOT Analysis
SWOT analysis is a technique for assessing the performance, competition, risk, and potential of a business, as well as part of a business such as a product line or division, an industry, or other entity. In this study, we identified our SWOT as shown in Figure 2-3.
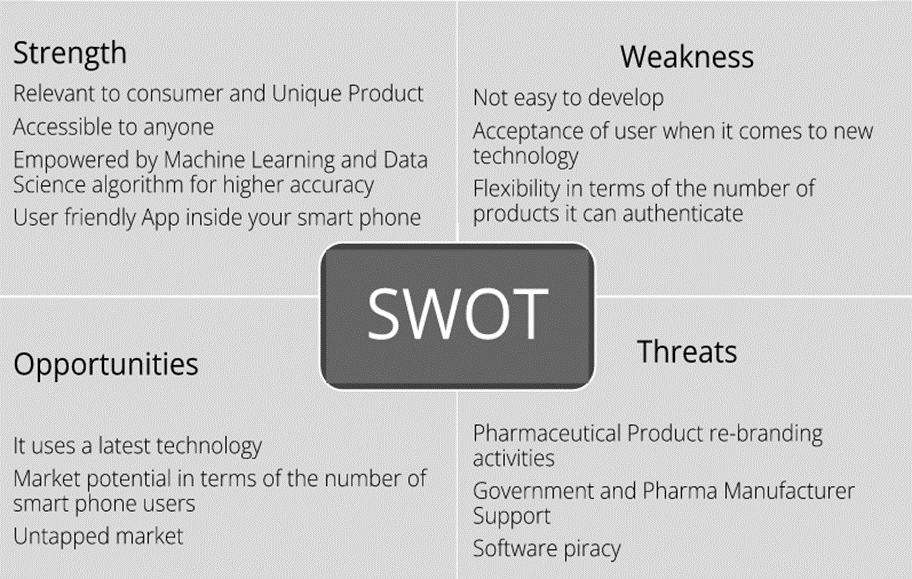
As of January 2023, there has been no available tool that can check the authenticity of pharmaceutical products (e.g. whether it conforms with the FDA standards or not), hence, making this our number one strength. Based on the TAM-SAM-SOM data, at least 7 million Filipinos already have a smartphone making it more accessible to everyone and a great number of potential market users.
Nowadays, data has been a key when it comes to decision-making. The product will use Machine Learning and Data Science algorithms to provide higher accuracy with the addition of cloud technology for faster data processing. Since there is no available tool right now, it will give a great opportunity and market potential.
In terms of weaknesses, one concern is the length of time required for end-to-end development. The process demands thorough capturing and defining of all parameters by the government to ascertain medicine authenticity, which may result in a longer development period. Additionally, there might be resistance from traditional users to adopting new technology, as some may find it challenging to use the tool. Surprisingly, based on our data, 60% of the surveyed population does not prioritize verifying the authenticity of their purchases. Lastly, the initial launch may face limitations in terms of product variety, as it would be confined to authenticating only the top 10 most purchased over-the-counter medicines.
As for the threats, rebranding might take place in a particular product every now and then. Since the application is very dependent on product packaging, a simple change in it might affect the accuracy of the application. The researchers are also looking at how much support the government and the pharmaceutical industry can offer to give us a buy-in on launching the app. Lastly, software piracy is very common, especially for newly launched products.
Market Validation
Medicine is indeed a necessity. Hence, it is also one of the most counterfeited products not just here in the Philippines but across the globe. Our motivation for this study was to empower the public, especially those who are in rural areas and cannot afford to purchase their medicinal needs in well-known pharmaceutical stores or those consumers who live in places where accredited pharmaceutical stores exist.
Currently, there are already more than 5 countries that have developed a similar product that aims to provide medical information to ensure the safety of the consumers.
In the Philippines, there is no existing application that helps the public determine whether a particular medicine conforms to the safety standards of the FDA or not. With over 7M smartphone users, this product has great market potential.
When we conducted our survey, we also asked our respondents if they would use the thing they described that would potentially help them in identifying the authenticity of the medicine. Of the results, 98.1% of the respondents answered YES whether it is free or paid. Out of those respondents who answered YES, 30% would only use it if it was free while 70% were willing to use it even if they had to pay for it.
With the existing problem, the potential users, and the interest of the public, we can say that this product has a great opportunity to succeed in the Philippine market.
| Total Respondent | 208 |
| Willing to use a tool to help them in authenticating their product | 204 |
| Yes, even if I have to pay for it | 142 |
| Yes, only if it is free | 62 |
About the Product

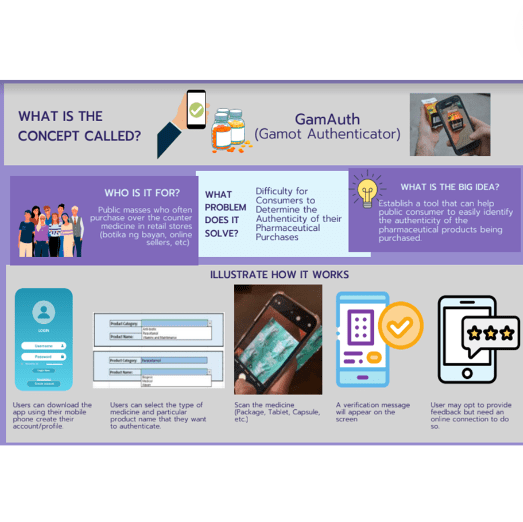
The GamAuth or Gamot Authenticator is a tool that can help public consumers easily identify the authenticity of the pharmaceutical products being purchased. This product is for anyone who has a smartphone and would want to verify the authenticity of the medicine purchased or the medicine for consumption.
For GamAuth to identify the type and authenticity of the medicine, it would apply the concept of the image classification algorithm which it would classify the type and authenticity of the medicine.
The initial phase of the application covered only the basic function of the tool which was to classify the scanned images and provide output according to the probability score percentage which ranges from 0-100, where 100% is the highest. Also, error handling was not yet incorporated into the tool. The users were required to use the tool in scanning the packages of those pharmaceutical products that were within our scope.
In the future, we plan to expand the scope of medicines included in our application by partnering and collaborating with pharmaceutical manufacturers. By establishing partnerships with these manufacturers, we can broaden our range of in-scope products. This will enable us to enhance our image classification capabilities, not only by analyzing packaging but also by detecting defects and anomalies in the medicines themselves. We will focus on attributes such as color, weight, size, and shape of the medicine.
By following this comprehensive plan, we can successfully expand our application’s scope, collaborate with pharmaceutical manufacturers, and enhance our image classification capabilities to provide a more comprehensive and accurate solution for our users.
The researchers will conduct thorough market research to identify reputable pharmaceutical manufacturers. They will evaluate the product portfolio, reputation, and quality standards of these manufacturers and then shortlist potential partners based on alignment with the project objectives. Once identified, the researchers will initiate communication with the selected pharmaceutical manufacturers and present the project vision, objectives, and potential benefits of collaboration. Negotiating partnership agreements, including the terms and conditions, will be the next crucial step.
To ensure seamless data integration and sharing with partner manufacturers, the researchers will develop a secure and standardized data-sharing mechanism. They will define data requirements, formats, and protocols, all while adhering to data privacy and security regulations.
As part of the project’s expansion, the researchers aim to collaborate with pharmaceutical manufacturers to include a wider range of medicines in the application. Specific criteria for product inclusion will be established, considering factors such as relevance, market demand, and safety considerations. To maintain the application’s accuracy, it will be regularly updated with new products and relevant information.
Additionally, the researchers plan to enhance image classification capabilities by researching and developing advanced algorithms. These algorithms will be trained to detect defects and anomalies in medicine packaging and attributes, such as color, weight, size, and shape. The accuracy and effectiveness of these algorithms will be validated through rigorous testing.
User feedback will be vital for continuous improvement. The researchers will establish a feedback mechanism to gather user input and suggestions, regularly analyzing and reviewing this feedback to identify areas for enhancement. The application will be iterated and updated based on user feedback and emerging industry trends.
Remaining compliant with regulatory requirements related to pharmaceutical products and data handling is of utmost importance. The researchers will stay updated with industry regulations and standards, implementing necessary measures to maintain data privacy and security.
To ensure the application’s effectiveness, it will be regularly monitored and evaluated by the researchers. They will analyze user engagement, satisfaction, and adoption rates. Additionally, the impact and effectiveness of the collaboration with pharmaceutical manufacturers will be measured, striving for continuous improvement and success.
How does the App work?
GamAuth is a user-friendly mobile application that utilizes a smartphone’s camera to enable users to verify the authenticity of pharmaceutical products easily. It caters to both Android and iOS devices, ensuring a broader reach among smartphone users. The application’s versatility allows it to function both online and offline, providing real-time data updates when connected and ensuring usability even in areas with limited internet connectivity. By employing advanced image classification algorithms, GamAuth can accurately differentiate between genuine and potentially counterfeit medicines, empowering consumers to make informed decisions about their purchases. Its simple and intuitive interface makes the authentication process seamless, making it a valuable tool in the fight against counterfeit pharmaceutical products and promoting public health and safety.
GamAuth represents a significant step towards protecting consumers from the risks associated with counterfeit medicines. Its compatibility across various devices and its capability to work offline cater to a diverse user base, offering a reliable solution even in areas with unreliable internet connectivity. The application’s commitment to accuracy through rigorous testing of image classification algorithms ensures that users can trust its results when validating the authenticity of pharmaceutical products. With GamAuth, consumers can have confidence in the medicines they purchase, enhancing their overall well-being and contributing to a safer pharmaceutical market.
User Interface Design and How the Application Works:

3.1.1.1 Installation procedure
- Download the application on your mobile phone via the App Store or Google Play. (Disclaimer: These will be the platforms in case it will be available for the public.)
- Users will have the option to create an account, or they can do it later, but this will not hinder them from using the application.
3.1.1.2 How to use the mobile application
- Open the application.
- A one-time terms and condition agreement checkbox will show for the user to read and agree before the use of the product. This includes the general disclaimer, “Ang produktong ito ay ginawa upang gabayan ang mga mamimili ng gamot kung sila ay hindi sigurado sa kalidad ng kanilang binibili. Hinihikayat pa rin namin ang mga gagamit na kumunsulta sa ekspertong pangkalusugan para sa tamang pagpapasya.”
- On the screen page, you will see two dropdown menus. The first one is for the type of medicine and the second one is for the brand name of the medicine. Select the type of medicine that you want to validate (e.g. Paracetamol, Antibiotic, Vitamins, Maintenance) then select the corresponding brand.
- A notification will prompt you to allow the application to access the camera on your smartphone. Select “Allow”.
- Your smartphone camera will open. Point it to the packaging of the medicine that you want to scan. In one to two seconds, a verification message will appear. Either the scanned medicine conforms with the FDA standards or not.
- if the scanned medicine conforms with the FDA standards, this message will appear on your screen: “Ang kalidad ng produktong ito ay garantisado”
- if the tool cannot confirm the authenticity of the scanned medicine, this message will appear on the screen: “Hindi namin masigurado ang kalidad ng produktong ito. Nasa inyo ang pagpapasya. Maaring kumunsulta sa eksperto o pinakamalapit na sentrong pangkalusugan (Health Center).”
- You can repeat the steps if you want to re-scan the product.
- Users are encouraged to provide feedback and ratings at the end. This will require the user to create an account to publish their feedback and ratings.
The application will base its distinction on the visual parameters of the medicine. Each medicine will have different parameters, depending on the manufacturer’s authenticity check standards such as but not limited to the tablet color (and layers, if applicable), the product pocket (if applicable), the foil pattern, the engraving (if applicable), the foil color, and the safety seal (if applicable).
In the development of GamAuth, below are the parameters followed by the team set by the manufacturer, United Laboratories, Inc (UniLab):
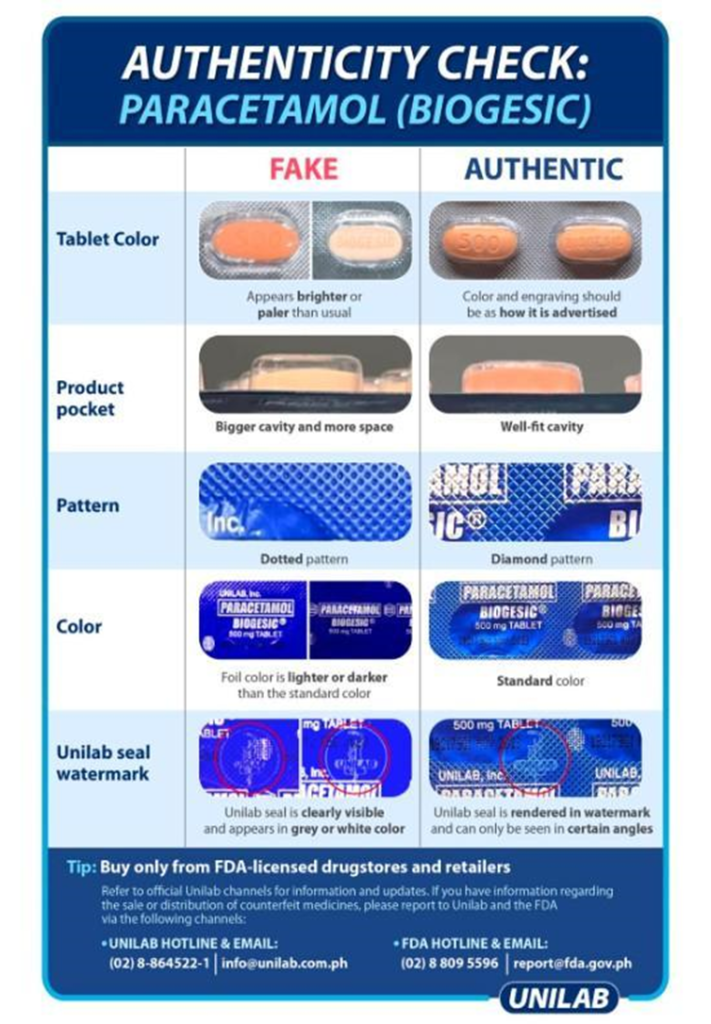
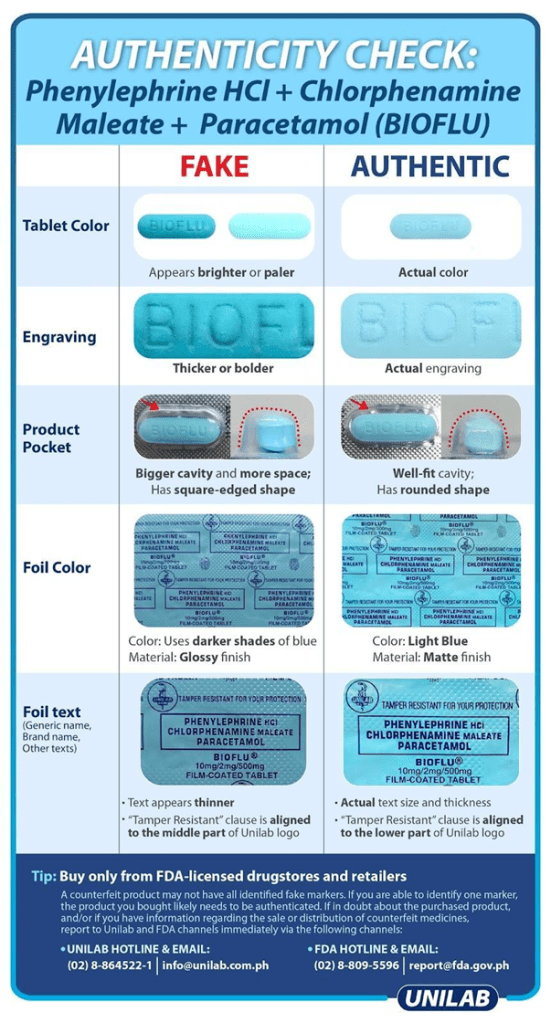
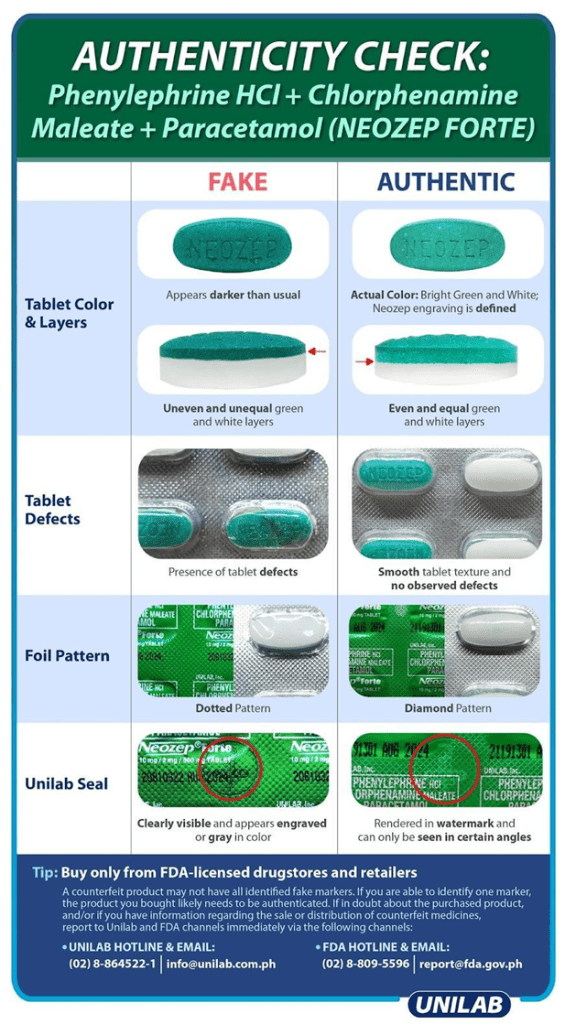
To explain further, FDA guidelines and Manufacturers’ advisories like the ones shown in Figure 3-2, Figure 3-3, and Figure 3-4, are already the standards to be used in identifying the authenticity of the specific medicines with or without the AI tools. Therefore, this must be used as the baseline visual parameters that any AI tool should consider as much as it is implementable and practicable. The details of preprocessing and processing these visual parameters are explained further in the Development portion of this paper.
Value Proposition
The product relieves the pains of the clients for it is automated, easily downloadable, has faster output, and is accessible on online and offline setups. The product creates gains for the clients since it displays real-time results using any smartphone, making it portable, user-friendly, and conforms with the FDA-approved standards.
Competitive Advantage
Customers will find several compelling reasons to choose our AI-enabled application for medicine authentication. GamAuth currently holds a unique advantage in the Philippine market, as it does not have any direct competitors. This sets it apart and provides customers with a distinctive solution that customers would not find elsewhere.
The prevalence of counterfeit medicines poses significant health risks, leading to mass poisoning and potential long-term health complications. The AI-enabled applications address this pressing issue by effectively detecting and preventing the circulation of fake medicines, ensuring the safety and well-being of consumers.
Moreover, GamAuth stands as the sole Deep Learning Image Classifier Artificial Intelligence Application for Medicine Authentication. This exclusivity makes our application the most dependable choice for authentication, as it offers cutting-edge technology and accuracy in distinguishing between genuine and counterfeit pharmaceutical products. With these significant advantages, customers can trust this application as the optimal solution for their medicine authentication needs.
Customers might choose other competitors over GamAuth for several reasons. For instance, there are already existing applications offering similar products in various countries, including Africa, India, Europe, and the Middle East. If these applications eventually become available in the Philippine market, they might be perceived as having better features or more advanced offerings compared to GamAuth. As a result, it becomes crucial for the researchers to continually enhance and innovate the application to maintain a competitive edge in the market and attract customers effectively.
Some potential customers may choose not to buy the product for a couple of reasons. Firstly, traditional customers may not favor this product as they often rely on their experience and intuition, rather than embracing the latest tools and technology. This preference for familiarity might lead the customers to opt out of using this application for medicine authentication.
Secondly, the technology exposure of certain customer segments could be a hindrance. Not all Filipinos are tech-savvy or enthusiastic about exploring the latest apps and technology. This lack of comfort with technology might deter some potential customers from using our AI-enabled application for medicine authentication. To address these challenges, it will be essential to educate and create awareness among customers about the benefits and ease of use our application offers, ensuring that even less tech-savvy users can confidently utilize it for authenticating their pharmaceutical products.
To ensure success in the future, the focus should be on generating public awareness and garnering support from both the government and pharmaceutical manufacturers. By fostering a widespread understanding of the importance and benefits of this AI-enabled application for medicine authentication, researchers can build a strong customer base and increase user adoption. Public awareness campaigns and educational initiatives will play a crucial role in achieving this goal.
Additionally, forging collaborative partnerships with the government and pharmaceutical manufacturers will be vital. Their support and endorsement of our application will not only enhance its credibility but also facilitate its integration into the healthcare system. Working hand in hand with these stakeholders will help overcome potential challenges and create a sustainable and impactful solution in the fight against counterfeit medicines. By prioritizing public awareness and fostering strategic partnerships, the researchers can pave the way for a successful future for our AI-enabled medicine authentication application.
In Figure 3-6, the competitor matrix is shown among GamAuth, PharmaSecure (PharmaSecure PAS India Private Limited, n.d.), and DrugXafe Mobile App (Tiga Healthcare Technologies, 2021).

Development
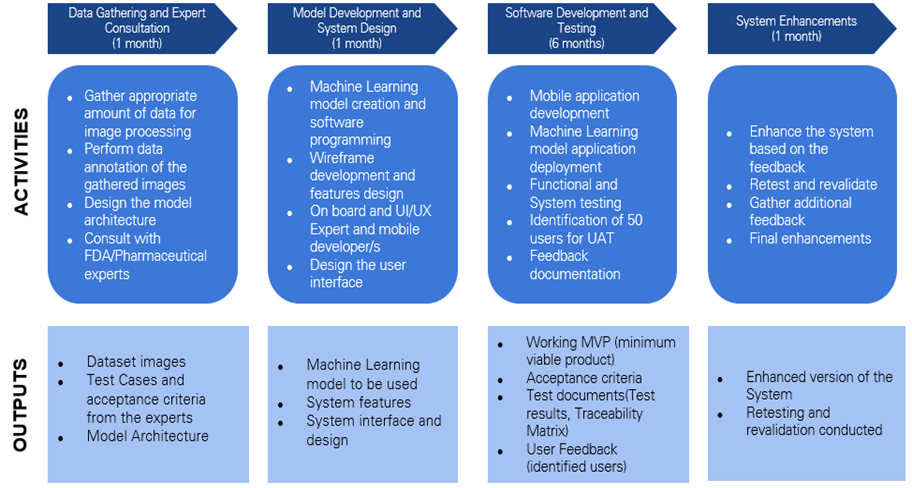
Product development strategy consists of the different methods and activities conducted in bringing new products or modifying existing products to the market. Figure 3-7 shows the product development strategy that comprises the activities in developing the GamAuth application.
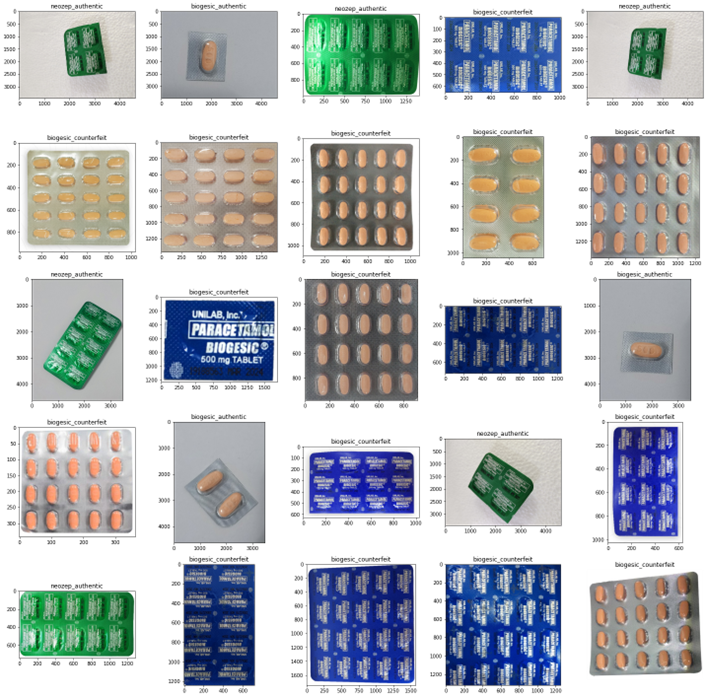
During the data-gathering process, the team requested images for both authentic and counterfeit medicines available from the Food and Drug Administration (FDA) through the Freedom of Information (FOI) portal. FDA has provided a series of advisory issues about counterfeit medicines to the public. These advisory issues(APPENDIX B) can be accessed through their website, and these contain several images of authentic and counterfeit medicines.

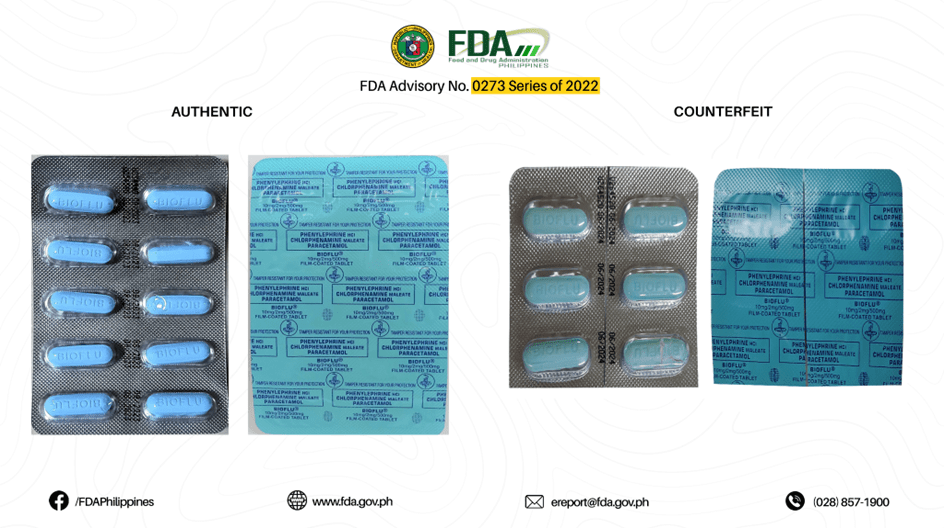
The authentic and counterfeit images of Biogesic, Neozep, and Bioflu were manually collected and scraped by the team to maintain the image resolution quality. The authentic and counterfeit images were separated and cropped from the FDA advisory and were stored in a folder separately. The folders were named “medicinename_authenticity” (i.e. neozep_counterfeit).

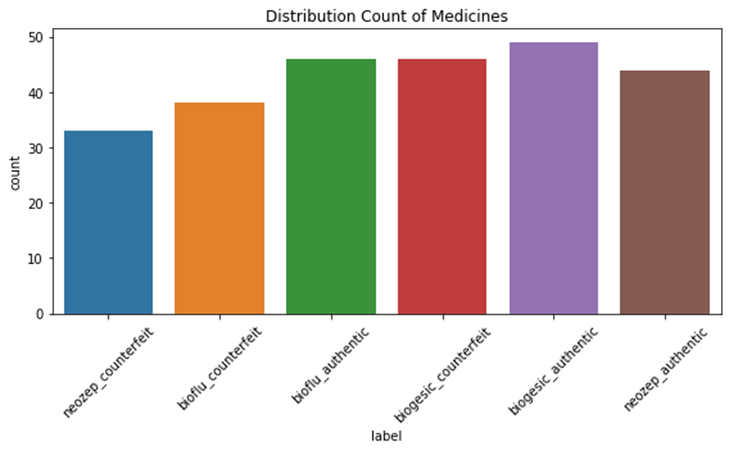
A total of 256 images (139 authentic and 117 counterfeit) were collected during the data collection process. The distribution of the data is shown in Figure 3-10. Biogesic had the most number of images while neozep_counterfeit had the least number of images.
The data were stored in GitHub and Kaggle datasets repositories for easier access whenever there would be a rebranding change with the medicine packaging that would require an update with the model.

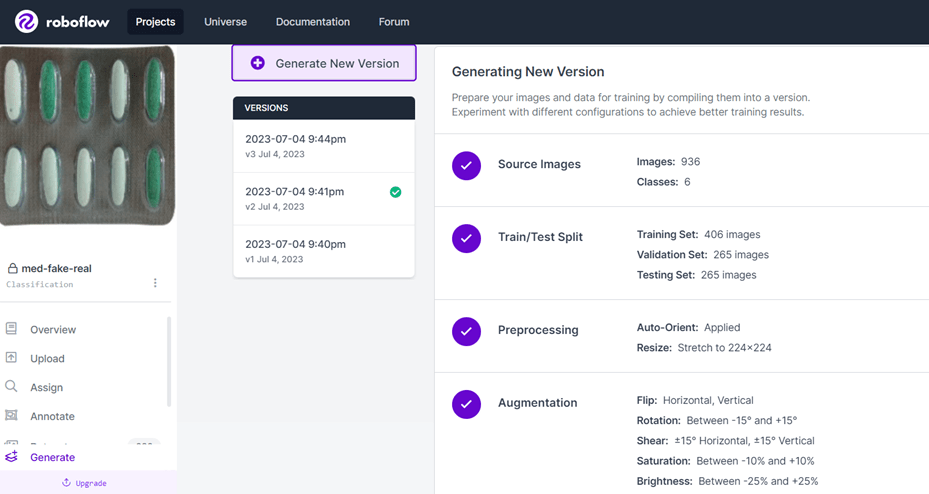
Data preprocessing played a significant role before building the model. It is a way to improve and enhance the quality of the images. The first data preprocessing technique was used to create different angle orientations of the images. The images were augmented and rotated to 90, 180, and 270 degrees.
Since the mobile application allows users to capture images of medicine in real-time, there are some instances where the lighting and environmental factors deviate from the quality of the images. These deviations may contribute an error to the classification process of the machine learning model. To avoid this issue, the Roboflow tool is used to apply the following data preprocessing and augmentation techniques to the images: resizing the image, vertical and horizontal flip, rotation, saturation, brightness, and exposure. Applying these processes makes the model more resilient to lighting, environmental factors, and camera setting changes. Table 3-1 shows the summary of these data preprocessing and augmentation techniques.
The images were divided into 3 categories, the train, test, and validation data. 70% of the images were used for training and 15% for validation. These were used during the training phase of the model. The remaining 15% of images were used during the testing phase, these would evaluate if our model was performing well with unseen images. Table 3-2 shows the final distribution count and size of the train, test, and validation data.
| Data Preprocessing | Data Augmentation |
| Resize: Stretch to 224×224 | Flip: Horizontal, Vertical Rotation: 90°, 180°, 270° Rotation: Between -15° and +15° Shear: ±15° Horizontal, ±15° Vertical Saturation: Between -10% and +10% Brightness: Between -25% and +25% Exposure: Between -10% and +10% |
| Image data | Size | Count |
| Train data | (1218,3) | 1218 |
| Test data | (265,3) | 265 |
| Validation data | (265,3) | 265 |
The image data was converted into batches of tensor image data by creating data generators using the ImageDataGeneration function in Tensorflow. Train datagen are based on the train images while valid datagen are based on the valid and test images. These datagen were fed into the deep learning model during development and evaluation.
GamAuth applied the concept of the Image Classification technique in classifying the type and the authenticity of the input medicine fed on the mobile application. Image Classification is a deep learning application where it identifies the associated labels to the images based on their certain features and characteristics. Labels were assigned to the training dataset which instructed the model what output was related to a specific image. The folders where the medicine is stored would serve as the labels of the images.
To obtain optimal performance, pre-trained models were used during model development. Pre-trained models were networks that were previously trained with large datasets and can be used to train on different data and for a similar/different task. Imagenet models were trained to imagenets dataset and can classify images into 1000 classes. The identified imagenet models used were Resnet50, EfficientNet, and InceptionV3. Previous studies about medicine and pill package classification used these models due to their performance and high accuracy such as the Automatic Drug Pills Detection based on Convolution Neural Network (Ou et al., 2020), the Automatic Medicine Identification Using a Deep Convolutional Neural Network (Hnoohom et al., 2018), and Classification of Philippine Herbal Medicine Plant Using EfficientNet on Mobile Platform (Mirandilla et al., 2022) for ResNet50, InceptionV3, and EfficientNet, respectively. These models were trained and evaluated to identify which is the best-fit model for the GamAuth application.
In training the models, all the layers except the output layer were frozen and were modified based on the input data. The new output layer consisted of Batch Normalization, Dropout, and Dense layers were added to the model. Adding a batch normalization layer would normalize the layer’s input which would make neural networks train faster and more stable through adding extra layers in a deep neural network. The dense layer consisted of a number of hidden layers (256 for EfficientNet while 512 for Inception and ResNet50) and a “ReLu” activation layer was added after the batch normalization layer. L1 and L2 Regularizers were included in this dense layer to reduce the complexity of the weights. Dropout layer with a rate between 0.2 and 0.45 was added that would turn off some of the neurons to prevent overfitting. The final layer was a dense layer with 6 neurons (the number of classes) and a sigmoid activation layer. Table 3-3 shows the summary and differences among the three models.
| Model Name | EfficientNetB0 | ResNet50 | InceptionV3 |
| Input Shape | (224,224,3) | (224,224,3) | (224,224,3) |
| Epochs | 40 | 40 | 40 |
| Batch Size | 64 | 64 | 64 |
| Added Layers | * Batch Normalization layer * Dense layer with 256 neurons, kernel regularizer, activity regularizer, bias regularizer and ReLu activation layer. * Dropout layer with 0.45 rate * Dense with 6 neurons and softmax activation layer | * Batch Normalization layer * Dense layer with 512 neurons * Dropout layer with 0.2 rate * Dense layer with 6 neurons and softmax activation layer | * Batch Normalization layer * Dense layer with 512 neurons * Dropout layer with 0.2 rate * Dense layer with 6 neurons and softmax activation layer |
| Optimizer | Adam | Adam | Adam |
| Learning Rate | 0.001 | 0.001 | 0.001 |
| Loss in compile | categorical_crossentropy | categorical_crossentropy | categorical_crossentropy |
| Metric in compile | accuracy | accuracy | accuracy |
Aside from adding Dropouts and Regularizers to the model, another way to avoid overfitting in the models was by incorporating callbacks such as Reduce Learning Rate on Plateau, and early stopping while the model was training. Reduce Learning Rate on Plateau or ReduceLROnPlateau is a Keras API callback where that reduces the learning rate by a certain factor when a metric has stopped improving. Early stopping is a method that would terminate the training once the model performance stops improving. Initially, the model started training with an epoch and learning rate of 40 and 0.001 respectively. The criteria handled and monitored by the callbacks were training accuracy until it reached 90%, once the training accuracy was at most 90%, callbacks would prioritize the validation loss, and the learning rate was adjusted by a factor of 0.5 where the new learning rate is equal to learning rate x 0.5, the learning rate was reduced every after two epochs of no improvement, training would end if there was no metric improvement after 5 consecutive adjustments of the learning rate, and every 20th epoch, a prompt message would appear and would ask the user to halt or continue to train the model by entering H or an integer.
After training the models, model evaluation was conducted on each model to identify the best candidate model for GamAuth. Since the GamAuth application would be dealing with medicine and health wellness, the criteria must meet the accuracy values ranging from 95% to 99%, loss value must be less than 0.1, every label must have a high value of f1-score, recall, and precision. The F1 score will ensure that there is no bias within the model and will be able to classify the labels. The precision and recall values will demonstrate the presence of false positive and false negative (type I and II errors) results within the model, therefore a high value will indicate the absence of these errors, and the model should have high accuracy and low loss so that it doesn’t overfit.
The plot of the training and validation performance of each model can be seen in Figures 3-15, 3-16, and 3-17. As the epochs increase, the validation (loss and accuracies) graphs tend to follow the trend of the training graph. By applying dropouts, regularizers, and callbacks, model overfitting was prevented.
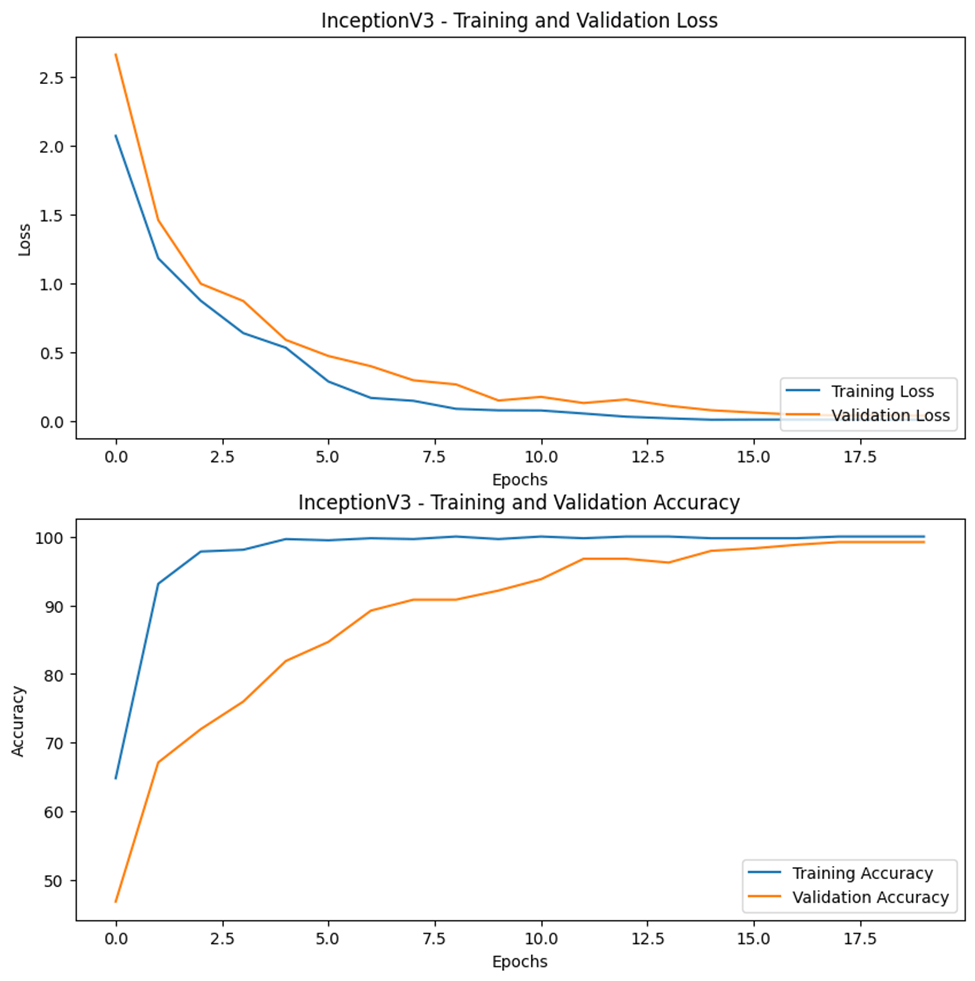

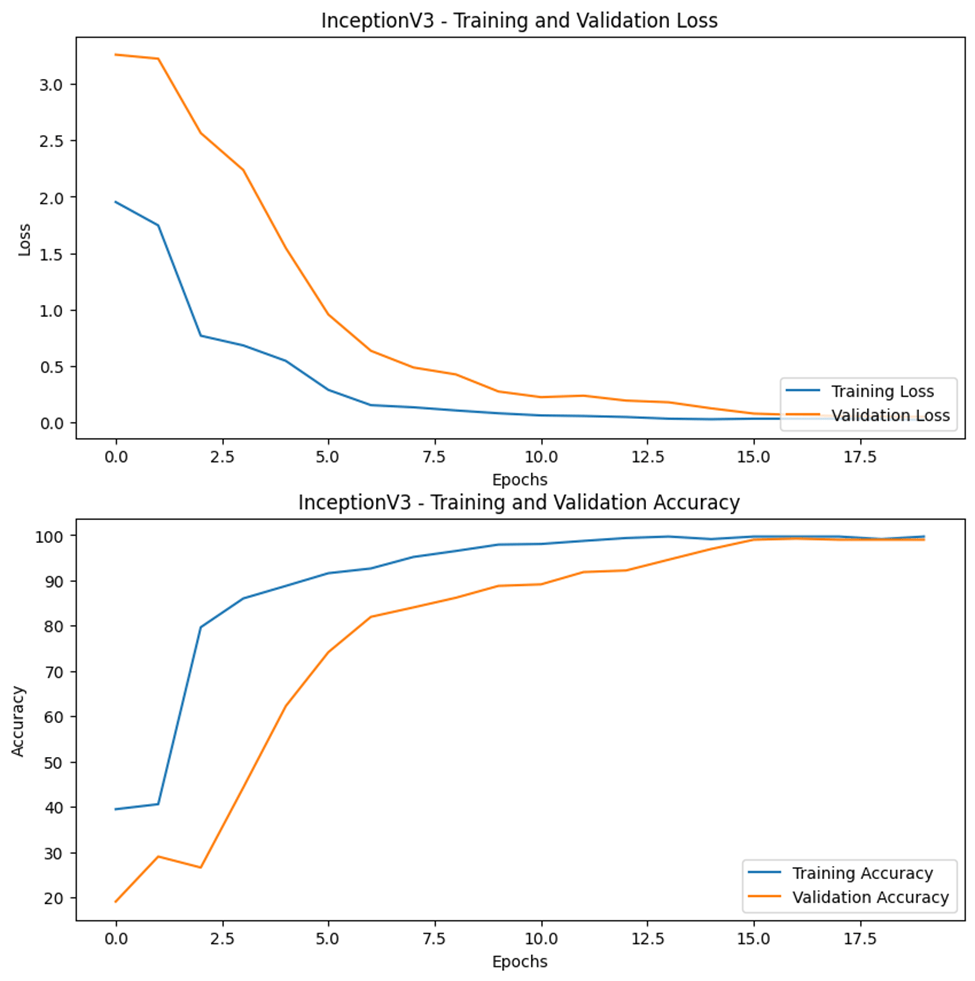
Table 3-4 displays the summary of the accuracies and loss for both the training and testing phases of each model. In terms of accuracy, all the models have a value greater than 95%. Among the three models, the ResNet50 model has the highest accuracy and least loss in both phases.
| Model Name | Training Loss | Training Accuracy | Testing Loss | Testing Accuracy |
| ResNet50 | 0.012 | 100 | 0.0438 | 99.01 |
| EfficientNetB0 | 0.365 | 98.592 | 0.3714 | 97.81 |
| InceptionV3 | 0.025 | 99.691 | 0.0594 | 98.82 |
Table 3-5 shows the Precision result of each model. Among the three models, ResNet50 has the highest precision value per label. The lowest precision for ResNet50 is the neozep_counterfeit label which has a value of 0.94.
| Labels | ResNet50 | EfficientNetB0 | InceptionV3 |
| bioflu_authentic | 1.00 | 1.00 | 1.00 |
| bioflu_counterfeit | 1.00 | 1.00 | 1.00 |
| biogesic_authentic | 1.00 | 1.00 | 1.00 |
| biogesic_counterfeit | 1.00 | 1.00 | 0.98 |
| neozep_authentic | 1.00 | 0.88 | 0.96 |
| neozep_counterfeit | 0.94 | 0.88 | 0.97 |
Table 3-6 shows the Recall result of each model. Almost all of the labels have a value of 1.00 per model except for neozep_authentic which has the least recall values. Among the three models, InceptionV3 and ResNet50 have the highest recall value for the neozep_authentic label.
| Labels | ResNet50 | EfficientNetB0 | InceptionV3 |
| bioflu_authentic | 1.00 | 1.00 | 1.00 |
| bioflu_counterfeit | 1.00 | 1.00 | 1.00 |
| biogesic_authentic | 1.00 | 1.00 | 1.00 |
| biogesic_counterfeit | 1.00 | 1.00 | 1.00 |
| neozep_authentic | 0.92 | 0.91 | 0.92 |
| neozep_counterfeit | 1.00 | 0.91 | 0.97 |
Table 3-7 shows the F1 score result of each model. Among the three models, ResNet50 has the highest f1 scores (equal or almost equal to 1) per label. The InceptionV3 model also did perform well since most of its f1 scores are above 0.9.
| Labels | ResNet50 | EfficientNetB0 | InceptionV3 |
| bioflu_authentic | 1.00 | 1.00 | 1.00 |
| bioflu_counterfeit | 1.00 | 1.00 | 1.00 |
| biogesic_authentic | 1.00 | 1.00 | 1.00 |
| biogesic_counterfeit | 1.00 | 1.00 | 0.99 |
| neozep_authentic | 0.96 | 0.88 | 0.94 |
| neozep_counterfeit | 0.97 | 0.91 | 0.97 |
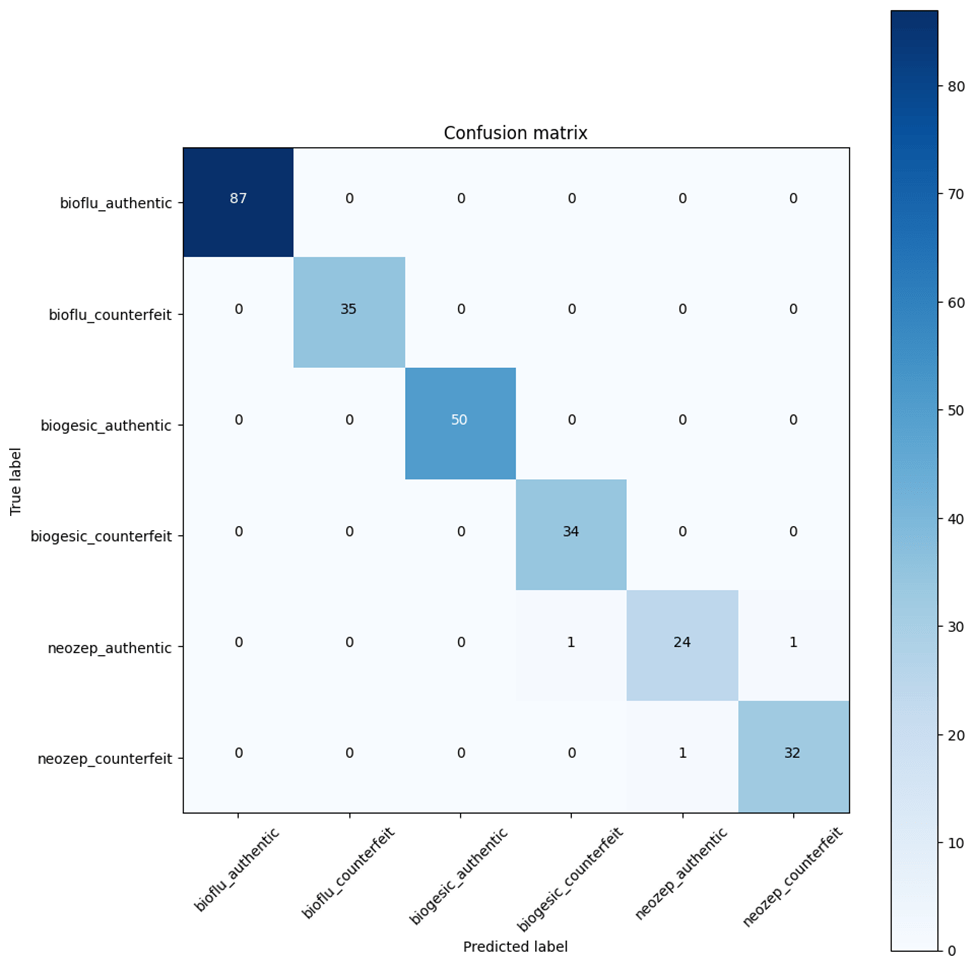
Figures 3-18, 3-19, and 3-20 present the confusion matrix of each model. Based on the heatmap plot, neozep is the type of medicine that has the most misclassified labels.


To select the final model, it was essential to consider multiple criteria, including high accuracy, low loss, high precision, recall, and F1 score. The objective was to identify the best model that meets all these requirements without compromising any of them. To achieve this optimization, the concept of Pareto efficiency or Pareto optimality was employed. Pareto optimality refers to a state where it is impossible to improve one criterion without negatively impacting at least one other criterion. By utilizing Pareto optimality, the selection process was optimized by identifying the models that lie on the Pareto front. These models provided the best possible performance across the multiple criteria, considering the inherent trade-offs between them. Rather than compromising on any specific criterion, Pareto optimality helps in identifying models that represent the optimal balance among the desired performance measures.
To perform Pareto optimality, the first step involved calculating the overall precision, recall, and F1 score for each model. This was achieved by obtaining the average precision, recall, and F1 score for each label within each model. Table 3-8 provides a summary of the metrics for each model.
| Model Name | Training Accuracy | Testing Accuracy | Training Loss | Testing Loss | F1-Score | Recall | Precision | |
| ResNet50 | 0.012 | 100 | 0.0438 | 99.01 | 0.99 | 0.99 | 0.99 | |
| EfficientNetB0 | 0.365 | 98.592 | 0.3714 | 97.81 | 0.96 | 0.97 | 0.96 | |
| InceptionV3 | 0.025 | 99.691 | 0.0594 | 98.82 | 0.98 | 0.98 | 0.98 | |
Next, weights were defined to facilitate the comparison between models. These weights were assigned based on the importance of each metric and the desired optimization direction. For example, a negative weight was assigned to training and testing loss metrics to minimize them, while positive weights were assigned to metrics like training and testing accuracy, F1 score, recall, and precision to maximize them. Table 3-9 shows the summary of weights used in the Pareto process.
| Metric | Weights | Reason |
| Training Accuracy | +1 | Maximize Training Accuracy |
| Testing Accuracy | +1 | Maximize Testing Accuracy |
| Training Loss | -1 | Minimize training loss |
| Testing Loss | -1 | Minimize testing loss |
| F1-Score | +1 | Maximize F1-Score |
| Recall | +1 | Maximize Recall |
| Precision | +1 | Maximize Precision |
Once the weights were defined, the next step was to iterate over each model and compare its metrics with all other models. This comparison involved assessing if the current model was dominated by any other model, meaning if there exists another model that performed better on at least one metric without performing worse on any other metric. If the current model was not dominated by any other model, it was considered Pareto optimal.
Upon applying the Pareto optimization process with the metrics data, it was determined that the ResNet50 model emerged as the optimal model. This implied that the ResNet50 model achieved the highest performance across the considered metrics, including precision, recall, and F1 score, while also balancing other important criteria such as training and testing accuracy, and training and testing loss. The ResNet50 model was used as the final model of the Gamot Authenticator application.
To deploy the model into mobile applications, the model was saved as a file compatible with the TensorflowLite framework. TensorflowLite is an open-source framework designed and developed by Google that would act as an interpreter between the model and the mobile device (Alsing, 2018). The model was converted into a file using Tensorflow Lite Converter so that it was able to load and run within the mobile application.
To further improve the performance of the model, the team recommended the to add more input data, especially to the images of Neozep, add more layers to the model, try other imagenet pre-trained models, and apply hyperparameter tuning such as Optuna or KerasTuner to optimize the parameters of the model.
Specification
The GamAuth application has three basic product specifications:
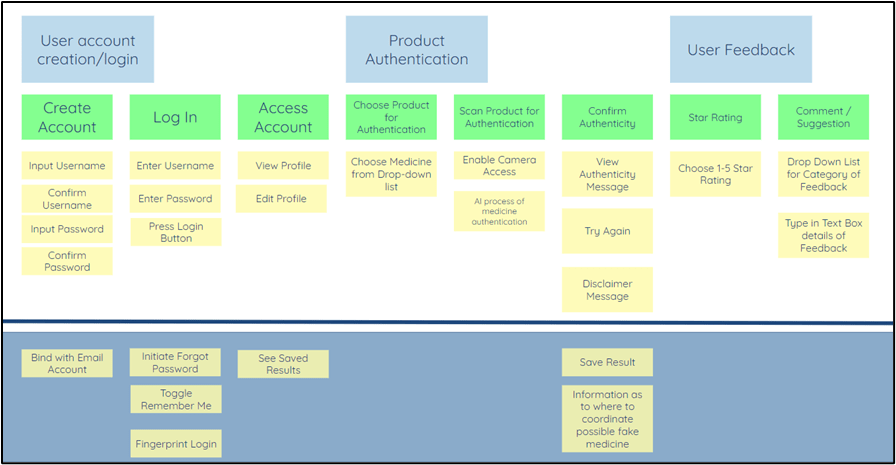
User Management. On this, users are required to input their personal information and create their login credentials. For our minimum viable product, we are only requiring our users to create their login credentials by simply providing their preferred username and password. Email authentication or one-time password authentication methods are not yet incorporated during this time. We also allow our users to manage their profiles.
Product Authentication. As discussed in the “How Does it Work” portion, our application requires the user to select the specific category of the medicine that they would like to scan. Furthermore, the user must allow the application to access its mobile phone’s camera for scanning purposes.
Machine learning algorithms are deployed in the backend of the application. This will ensure a high level of accuracy in determining if the scanned product adheres to the FDA standards and is not a counterfeited product.
The results will be displayed on the screen of the mobile phone in less than 2 seconds.
User Feedback. To continuously improve our product, we encourage our users to rate our applications. We will be asking for comments and recommendations as well, but these are not required.
Using this feature will require the user to be connected online. The results will be then shared with us in the form of a Google sheet.
See Figure 3-21 for the illustration of product specifications.
Validation
The BitWagon performed a discussion with some of our respondents regarding the development of the GamAuth application in addressing the defined problem. In summary, most of the respondents are excited to see how we’ll be able to execute and implement this solution. However, there are some that still have hesitations when it comes to the product due to different factors like government approval, manufacturing considerations, and our business model. In the end, they all agreed that there is a great opportunity for this product since there is no existing solution to the current problem related to counterfeit medicines. Some of the notable feedback is shown in Table 3-10.
| Name of Person/s Interviewed | Feedback about the Solution |
| Mr. Mishael Mark C. Parocha – One of the respondents in the public survey conducted last 28 February 2022. | Non-verbatim: “The concept is exciting. Will you also consider generic medicines? What will happen if the manufacturer changes its packaging?” |
| Discord Community – Group where the majority responded in the public survey conducted last 28 February 2022. | Consolidated feedback: “Looking forward to this solution. We are hoping for its success.” |
| Mr. Lixtel Rubis – A respondent from the public survey conducted last February 2022. | “Will it cover all types of pharmaceutical medicine or a specific group such as over-the-counter drugs?” |
| Ms. Ivy E – A respondent from the public survey conducted last February 2022. | “The idea is cool and useful to the public masses, however, I’m quite curious regarding the solution. What if I only bought 1 piece of medicine, will it scan the box, mat, or medicine itself? ” |
| Nestor Tarong Jr – Online shopper | “It’s a great idea. However, it has to be recognized by FDA/government as a legitimate tool to check the validity of the product. Otherwise, it will not make any sense to the user.” |
| Dimple Roque – Entrepreneur, small pharmacy owner. | “In my years in the business, I don’t recall anyone suffering because of consuming a fake medicine. Hence, I don’t see it as a problem.” |
| Charlene Jennica Collado – Business Coach | “How are you going to sell/monetize this idea?” |
| Ben Lim – Marketing Manager | “If your target market is those in the rural areas, then most of them are not smartphone users” |
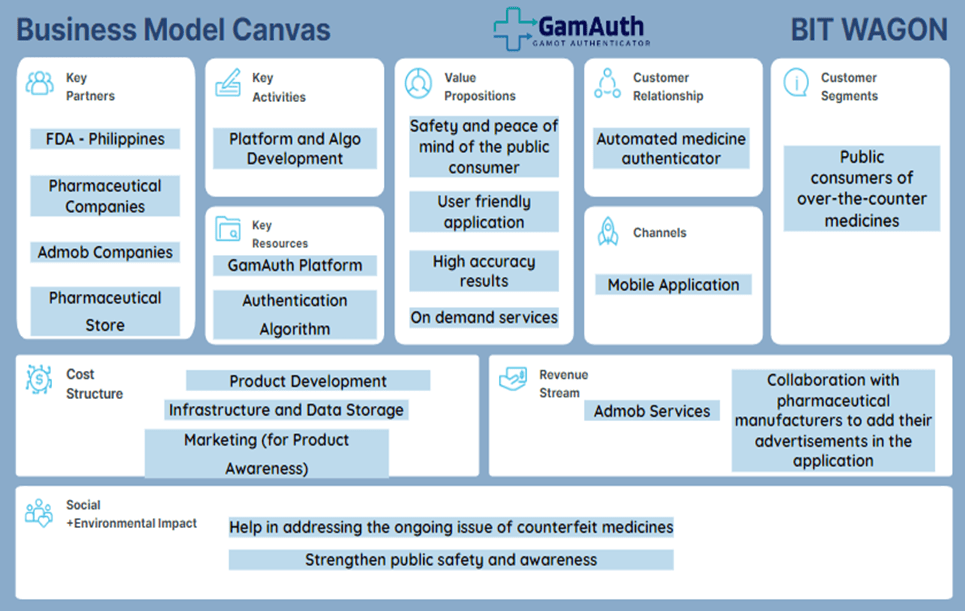
Business Model Canvas
The mobile application will be available to the public, aiming to generate profits and ensure sustained subscriber loyalty. To achieve this, various revenue streams will be explored, including paid advertisements and revenue-sharing partnerships.
Paid advertisements, the application has designated space for advertising and collaborating with different brands to feature their ads. Implementing the Google AdSense model will facilitate seamless transactions and payment options.
Simultaneously, revenue-sharing partnerships will be pursued with pharmaceutical manufacturers. By offering them free advertisement space on our application, the expectation is to receive endorsements from these manufacturers to their distributors and retailers. This strategic approach aims to accelerate product awareness and attract additional brands to advertise on our platform. In terms of revenue sharing, we propose a 50/50 split with our partners to foster mutually beneficial outcomes.
In Figure 4-1, you can see the visual representation of our building blocks.
Go-To-Market Strategy
GamAuth’s target audience comprises smartphone users residing in rural areas of the Philippines, who frequently engage with social media and enjoy shopping online. They are individuals who purchase over-the-counter medicines from retail stores or small “botika” shops. Additionally, these audiences include wholesale buyers of over-the-counter medicines or small pharmaceutical business owners. The age range of our target audience is 18 years and older. These individuals represent a diverse group with a common interest in utilizing our mobile application for authenticating pharmaceutical products and ensuring their purchases are genuine and safe.
The marketing campaign strategy will leverage the influential reach of social media to raise awareness about the pressing issue of counterfeit medicines. Testimonials from the FDA, individuals affected by counterfeit medicines, pharmaceutical companies, and law enforcement will be collected to emphasize the urgency of finding a robust solution. Social media platforms will serve as a showcase for our mobile application’s benefits, with informative content on how to use it, FAQs, and straightforward advertisements for the public.
In rural areas, collaboration with influencers helps to disseminate information about the issue and its potential health risks through engaging social media platforms. This approach aims to inform and engage the community in understanding the problem and the significance of using our mobile application to authenticate pharmaceutical products effectively. By harnessing the power of social media, the intent is to create a strong and impactful marketing campaign that resonates with the target audience, encouraging them to prioritize their health and safety by using this application.
The marketing campaign strategy also involves forming partnerships with health centers in rural areas or places where major pharmaceutical distributors are not present. These health centers will play a crucial role in informing the community about the existing issue of counterfeit medicines and promoting our product as an effective solution. To achieve this, flyers and posters will be created and displayed in the vicinity of health centers, ensuring public visibility and awareness. These materials will highlight the problem of counterfeit medicines, the potential health risks they pose, and how the solution can address these concerns. By leveraging health centers as key allies, the goal is to reach and engage the target market effectively, empowering them with the knowledge and means to protect their health and well-being through this mobile application for medicine authentication. Furthermore, partnerships with government agencies such as the FDA, Customs, and PNP will be established. As there is currently no existing solution to the problem of counterfeit medicines, there is a great potential that the tool’s ability to inform the public about the authenticity of medicines in line with FDA standards.
Business Operation
Our business operation model is divided into three pillars:
| Department | Description/Responsibilities |
| Technology Department | This department holds a range of key responsibilities, including product development, deployment, and cyber security. Their duties also encompass selecting the most suitable tools, infrastructure, and software required for operations. Moreover, they serve as the business’s technical experts, providing valuable expertise in their domain. |
| Commercial Department | This department takes on vital roles in business operations, marketing, and research. They hold the responsibility of ensuring successful market penetration for our application and are driven by the primary goal of revenue generation. |
| Financial Department | This department plays a pivotal role in overseeing the business’s financial management, covering both operational and capital expenses. Additionally, they are responsible for addressing legal and administrative requirements. Their main focus centers around ensuring business compliance and providing support to the company’s core functions. |
Please refer to Table 6-2 below for the detailed activities and responsible units.
| Number of advertisements appears on the application Number of clicked advertisements Revenue generated | Personal Unit/ Responsible | Performance Indicator | Timing | Resource Requirement |
| Model Development Training, Testing, Maintenance of ML Model | Data Science and Machine Learning Team (AI Developers) | New types of medicines trained in the model Maintain high accuracy of the model | Quarterly | Computer, Internet, Annotation Services, Cloud Server, Databases |
| Product Development Software Development, Testing and Deployment Software Related Knowledge Transfer and Handover | Outsourced Software Development Team (Mobile Developer, QA Tester, UI/UX Expert) | Working application Trained product development personnel Software Documentation | Quarterly (for Year 0) | Laptop, Mobile, Internet, Databases, Cloud Server |
| Software Feature Development and Maintenance | Product Development Team (Project Developers, Software Engineers) | New features in the application Less reported issues/bugs High customer satisfaction rating | Quarterly (after Year 0) | Laptop, Mobile, Internet, Databases, Cloud Server |
| Marketing Research | Marketing Research Team | Number of application downloads Number of pharmaceutical partners | Quarterly | Laptop, Internet, Marketing funds,Marketing Software Tools |
| Sales Monitoring | Sales Team | Number of advertisements appear on the application Number of clicked advertisements Revenue generated | Quarterly | Laptop, Internet, Sales Software Tools |
| Administration, Finance, and Support | Administration Team | Budget Utilization Business Operation Processes Legal and Permits Processes | Annual | Office Supplies, Funds |
One of the milestones that can determine the success of GamAuth is the number of users. In year 0, we will be focusing on marketing our product, strengthening our brand, and looking for investors. Hence, in Table 6-2, you will see that there is no projected number of users for the year 0.
Furthermore, our plan is to launch our product in the targeted market first as a pilot. Because of that, we are not expecting a full blast of users for the first 5 years.
Please see Table 6-3 for the projected number of users year by year.
| Year | Number of Users |
| 0 | 0 |
| 1 | 2000 |
| 2 | 3000 |
| 3 | 4500 |
| 4 | 8875 |
| 5 | 15532 |
Financial Plan
The GamAuth will require Php 1,857,000.00 capital investment in the initial product development year (Year 0) which covers UI/UX outsourcing, software subscriptions, office equipment, salaries, communication and transportation expenses, and legal consultations. For the first 2 years of the business operation, a capital investment of Php 393,000.00 and Php 319,000.00 for Year 1 and Year 2, respectively, are needed to cover the expenses exceeding the expected revenue for the said years.
For the preceding years, we are expecting a sustainable business that can cover the operating expenses and capital expenses from its revenue. In Table 6-4, detailed information on the expected expenses are shown.
| Expenses | Year | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Outsourcing | ||||||
| Mobile Developer @ P30,000 in 6 months | ₱180,000.00 | |||||
| UI/UX Expert @ P15,000 in 1 month | ₱15,000.00 | |||||
| QA Tester (1) @ P15,000 in 2 months | ₱30,000.00 | |||||
| QA Tester (2) @ P15,000 in 2 months | ₱30,000.00 | |||||
| Annotation Services | ₱7,000.00 | |||||
| Subscription | ||||||
| Google Cloud Subscription | ₱36,000.00 | |||||
| Roboflow | ₱14,000.00 | |||||
| Equipment | ||||||
| Laptop Computer | ₱50,000.00 | ₱50,000.00 | ||||
| Android Cellphone | ₱40,000.00 | ₱40,000.00 | ||||
| IOS Cellphone | ₱60,000.00 | ₱60,000.00 | ||||
| MOOE | ||||||
| Communication Expenses | ₱5,000.00 | ₱5,000.00 | ₱5,000.00 | ₱5,000.00 | ₱5,000.00 | ₱5,000.00 |
| Transportation Expenses | ₱10,000.00 | ₱10,000.00 | ₱10,000.00 | ₱10,000.00 | ₱10,000.00 | ₱10,000.00 |
| Paid Resources | ||||||
| Paid images | ₱20,000.00 | |||||
| Salary | ||||||
| Project Developer (1) | ₱420,000.00 | ₱420,000.00 | ||||
| Project Developer (2) | ₱420,000.00 | ₱420,000.00 | ||||
| Project Developer (3) | ₱420,000.00 | ₱420,000.00 | ||||
| AI Developer | ₱504,000.00 | ₱504,000.00 | ₱504,000.00 | ₱504,000.00 | ||
| Operation Chief | ₱504,000.00 | ₱504,000.00 | ₱504,000.00 | ₱504,000.00 | ||
| Finance and Admin Chief | ₱504,000.00 | ₱504,000.00 | ₱504,000.00 | ₱504,000.00 | ||
| Finance and Accounting Personnel | ₱420,000.00 | ₱420,000.00 | ₱420,000.00 | ₱420,000.00 | ||
| Marketing Personnel | ₱360,000.00 | ₱360,000.00 | ₱360,000.00 | ₱360,000.00 | ₱360,000.00 | |
| Legal and Admin Personnel | ₱420,000.00 | ₱420,000.00 | ₱420,000.00 | ₱420,000.00 | ||
| Software Engineer (Product Maintenance) | ₱480,000.00 | ₱480,000.00 | ₱480,000.00 | ₱480,000.00 | ₱480,000.00 | |
| Logistics and Marketing | ||||||
| Marketing and Advertising Expenses | ₱100,000.00 | ₱20,000.00 | ₱20,000.00 | ₱20,000.00 | ₱100,000.00 | |
| Taxes and Permits | ₱50,000.00 | ₱50,000.00 | ₱50,000.00 | ₱50,000.00 | ₱50,000.00 | |
| Consultation (Legal/Accounting) | ₱100,000.00 | ₱100,000.00 | ||||
| TOTAL | ₱1,857,000.00 | ₱2,365,000.00 | ₱3,277,000.00 | ₱3,427,000.00 | ₱3,277,000.00 | ₱3,357,000.00 |
Achieving each milestone in getting the number of users per year is equivalent to monetary value. By the rate shown in Google AdSense, per click amounts to $ 17.00. In addition, a paid advertisement and collaboration with pharmaceutical companies are targeted to earn revenues.
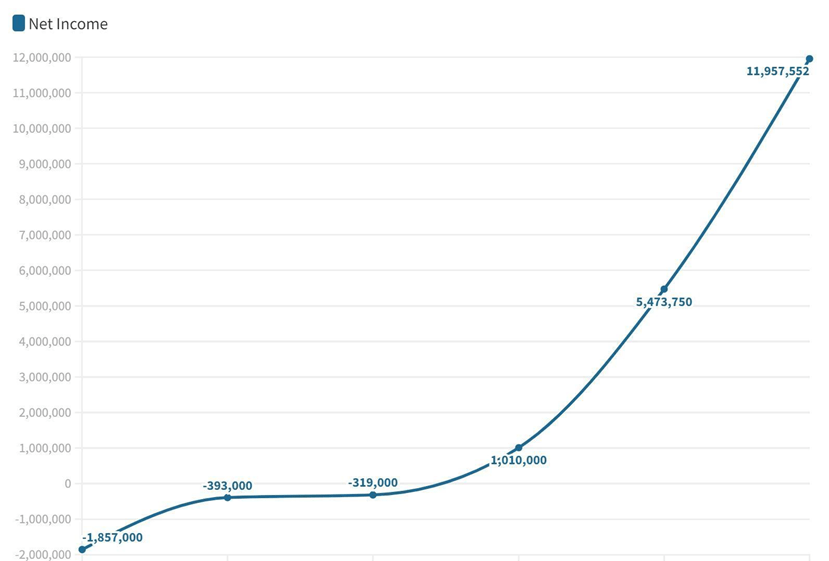
In Year 1, GamAuth is expected to earn at least PhP 1,972,000.00 with 2000 active users, which resulted from a comprehensive marketing drive of the product. In Year 2, we expect a lesser number of new users but still expect to earn PhP 2,958,000.00 with 3000 active users.
Continuous marketing efforts and collaboration with pharmaceutical companies will help us earn at least PhP 4,437,000.00 in Year 3 and are expected to grow until Year 5. Table 6-5 shows the detailed information on the Expected Number of Users, Revenue, and Expenses.
| Year | Number of Users | Revenue | Expenses |
| 0 | 0 | 0 | ₱1,857,000.00 |
| 1 | 2000 | ₱1,972,000.00 | ₱2,365,000.00 |
| 2 | 3000 | ₱2,958,000.00 | ₱3,277,000.00 |
| 3 | 4500 | ₱4,437,000.00 | ₱3,427,000.00 |
| 4 | 8875 | ₱8,750,750.00 | ₱3,277,000.00 |
| 5 | 15532 | ₱15,314,552.00 | ₱3,357,000.00 |
In Figure 6-1, the net income projection shows that as we meet and achieve the target market and goals, we will be starting to generate income by Year 3.
Risk Management
Figure 6-2 shows our Risk Management Matrix. We identified three major risks for our business wherein one is high risk and two medium risks.
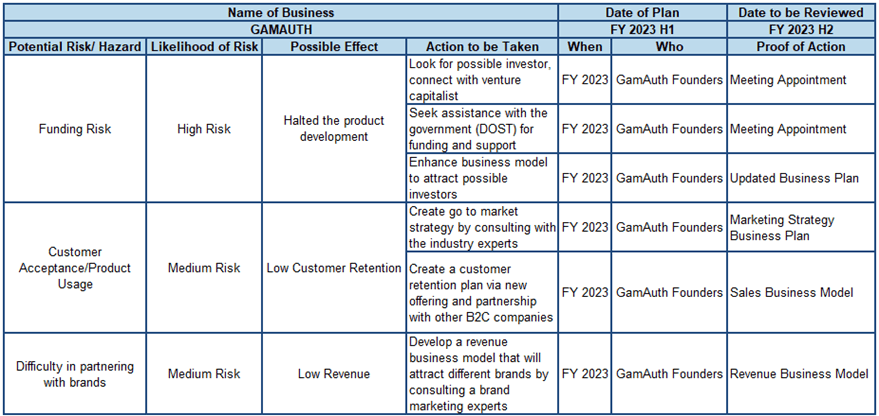
Funding Risk. The initial estimated budget for product development is half a million pesos (P500,000.00). It is already a huge amount to launch our minimum viable product. Also, the projected expenses in our first year would be more than P1.8M. Hence, we defined this as the number one risk.
As a mitigation plan, the team needs to find a possible investor through venture capitalist companies. The team is also exploring the possibilities of getting funds from our government, especially the Department of Science and Technology (DOST).
Customer Acceptance/Product Usage. Convincing target customers to use a product is always a challenge for a newcomer in the industry. Even though there is an existing problem that can be addressed by our mobile application, acceptance of the users is still a risk that we need to address.
As a mitigation plan for convincing the target market to use the product and the things that need to be taken into consideration to ensure retention of the users, the researchers are looking for a strong go-to-market strategy by consulting industry experts, as well as exploring the possibility of partnering with the Business to Customer (B2C) industry for deployment plans.
Difficulty in Partnering with Brands. Since the main source of revenue will come from advertising and brand partnerships, the researchers need to convince them to tie up with the business. However, it will be a challenge for a new player in the industry. As for the mitigation plan, the researchers need to have a better revenue business model to attract investors and partners. This can be achieved by consulting experts in industries. Exploring the possibilities of doing a shared revenue model with advertising brands, especially those in the pharmaceutical industry is another option.
About the Authors
The authors are graduates of Professional Science Master’s Degree in Data Science (PSMDS) at Technological Institute of the Philippines – Quezon City.
The authors call their group, “Bit Wagon”.
Bit Wagon is a metaphorical concept that combines the technological term “bit” with the societal notion of a “wagon” to create a unique and compelling image. Let’s break down the components:
- Bit:
- In the context of programming and computer science, a “bit” is the smallest unit of data in computing and digital communications. It can represent a binary digit, which can have one of two values: typically 0 or 1. Bits are fundamental to all digital technology, forming the basis for encoding information and executing computational processes.
- Beyond its technical definition, a bit symbolizes the essence of digital innovation, progress, and the transformative power of technology. It embodies the core principles of computing, including precision, efficiency, and versatility.
- Wagon:
- In society, a “wagon” traditionally refers to a horse-drawn vehicle with four wheels, historically used for transportation, commerce, and migration. Wagons played a crucial role in the development of human civilization, facilitating the movement of goods, people, and ideas across vast distances.
- Metaphorically, a wagon represents mobility, connectivity, and collective endeavor. It embodies the spirit of exploration, adventure, and community cooperation. Wagon trains, in particular, evoke images of journeys, teamwork, and shared aspirations.
- Combining “Bit” and “Wagon”:
- Bit Wagon merges the concepts of digital innovation and societal progress into a single, evocative image. It symbolizes the fusion of technology and human endeavor, where digital advancements propel societal transformation and vice versa.
- Just as a wagon carries goods and passengers along a journey, Bit Wagon carries the digital infrastructure, knowledge, and aspirations of a community toward shared goals and destinations.
- Moreover, Bit Wagon embodies the idea of inclusivity and collaboration in the digital age, where individuals and organizations come together, like passengers on a wagon train, to harness the power of technology for collective benefit and progress.
In essence, Bit Wagon is more than just a name; it’s a symbol of innovation, connectivity, and collective endeavor in the ever-evolving landscape of technology and society.
The following are the authors of GamAuth and the members of Bit Wagon:
| ARCHIBALD L. ECHANO, REE, PSMDS Principal Engineer A, Sub-transmission Management Department National Transmission Corporation (TransCo) qalechano@tip.edu.ph |
| ALFIE M. ECONG, ECE, PSMDS Head, Business Intelligence and Data Analytics DITO Telecommunity Corporation qamecong@tip.edu.ph |
| ROCELLE NATHALIE V. ONG, ECE, ECT, CTFL, PSMDS Machine Learning Developer, ANZ Banking Group, Ltd. qrnong@tip.edu.ph |
Disclaimer
GamAuth, as described herein, is a proposed idea and concept developed by Bit Wagon. It has not been fully implemented or executed at this time. The information provided regarding GamAuth, including its functionality, features, and potential benefits, is based on conceptualization and hypothetical scenarios.
Bit Wagon makes no guarantees or representations regarding the availability, performance, or functionality of GamAuth, as it has not yet been developed or deployed. Any references to specific capabilities or outcomes are speculative and subject to change.
This document serves as a proposal and exploration of the GamAuth concept and does not constitute a commitment to develop or release the described technology. Bit Wagon reserves the right to modify, revise, or abandon the GamAuth concept at any time without prior notice.
The inclusion of GamAuth in any discussions, presentations, or communications does not imply its current existence or availability for use. Any expressions of interest, feedback, or collaboration related to GamAuth are welcomed but do not constitute a binding agreement or commitment.
Bit Wagon disclaims any liability for reliance on the information provided herein regarding GamAuth, as it is presented for informational and discussion purposes only. Individuals or entities considering involvement with GamAuth are encouraged to conduct their own due diligence and seek professional advice as appropriate.
Thank you for your understanding and interest in our proposed idea, GamAuth.
Copyright © 2023 Bit Wagon. All rights reserved.
GamAuth, including its idea and concept, is the intellectual property of Bit Wagon. Any reproduction, distribution, or unauthorized use of GamAuth, its idea, or concept, in whole or in part, without the express written permission of Bit Wagon, is strictly prohibited.
This copyright applies to all forms of media and communication, including but not limited to digital platforms, printed materials, presentations, and promotional materials.
For inquiries regarding the authorized usage or licensing of GamAuth, please contact the author of kashikoiph or any of the members of the Bit Wagon.
Unauthorized use of GamAuth, its idea, or concept may result in legal action to protect Bit Wagon’s intellectual property rights.
Thank you for respecting our intellectual property.
Leave a comment